Sourdough Starter – Evolution of Microflora

It is astounding how a mixture of flour and water evolves into a bubbly active sourdough starter in a matter of days. This is the result of the fermentation of the two basic components, flour, and water. This leads to the establishment of sourdough starter and the evolution of sourdough microflora! Sourdough starter provides conducive habitats for microbes. As a result, it can support the growth of over 50 species of lactic acid bacteria and more than 20 species of yeast.
I am going to elucidate the connection between the creation of sourdough starter, the evolution and establishment of the microflora. I will support it with my recent experiment with starters!
Experiment with Sourodugh Starter Creation
I set out on a task to find out which of TWF’s whole wheat flours creates the most stable and viable sourdough starter. However, I ended up with four unique ‘baby’ starters with varying characteristics – rise, aroma, flavor, and texture (Fig 1). My stint also generated a mound of observations and data. These observations are validation of the facts we have known as sourdough bakers.
I followed my protocol for starter creation, which involves starting off the process with a mixture of flour and water. This is followed by backslopping every 24 hours for the first 7 days or until a fairly stable starter is obtained. I also measured the pH on each day just before the refreshment using litmus papers.

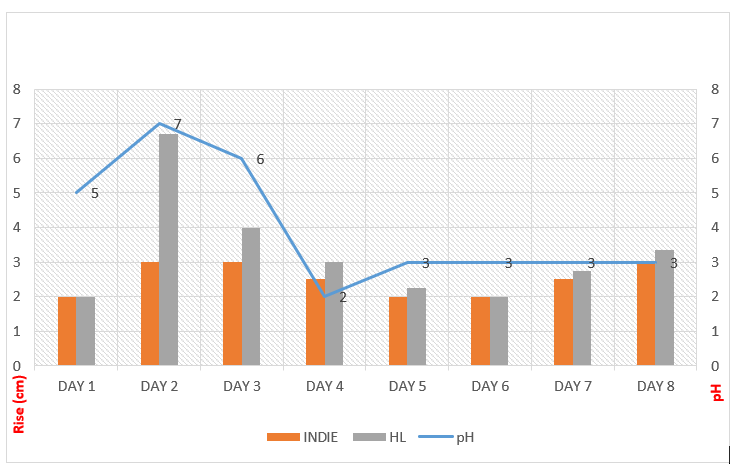
Day 1 was fairly silent with a little rise and a pH of 5. But, on Day 2 the starter seemed to be on steroids. interestingly, the pH was at 7 (basic!) (Fig. 2). The starters smelled of rotting fruits or vegetables and seemed discolored and slimy.

The activity in the starter fell through days 3, 4, and 5. I observed a consistent fall in pH too. The activity in the starter was at a standstill and seemed like the starter has gone bad or died! However, the starter kept getting more acidic with each passing day. Most importantly, Day 5 was monumental. Activity in starter picked up and the pH stabilized at 3. pH of 3 is typical to mature starters. Day 7 and day 8 showed a consistent rise and stable pH readings. This indicated, the successful creation of the starters (Fig. 3)!
Fairly simple!
Actually not! Sourdoughs are considered extremely complex ecosystems. LAB and yeasts represent the prevailing microflora. Complex interactions within the starter microorganisms and between the starter and the contaminant microflora of the sourdough occur, when a starter culture is established! Yes, there is contaminant microflora and which is why we observe a dramatic activity on the early days of starter creation. The starter actually rots and goes through a spoilage phase before it kicks back and stabilizes. And most importantly, it is very difficult to get a well propagated and established starter culture contaminated ever again!!
But how does that happen?!
In the initial days of starter creation
Let’s take a step back. Cereal grains are naturally contaminated by eucaryotic (molds and yeasts) and procaryotic (bacteria) organisms. The microbes can follow into the flours and thus be found in products made with flour, such as sourdoughs. As flour and water are mixed in a bowl, almost immediately yeast and bacteria from the atmosphere and the flour itself begin feeding on the sugars in the flour. At first, just about any microbe can grow on this rich energy source which includes spoilage bacteria too.
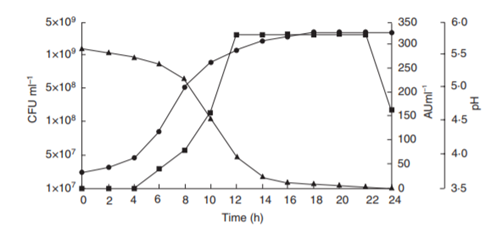
Subdominant LAB such as Enterococcus faecium and Pediococcus pentosaceus are stronger acidifiers than Lactobacillus sanfranciscensis at the beginning of sourdough production. Those species that, inhibit indigenous microorganisms other than LAB by lowering the pH, might prepare the environment for the establishment of the typical species (e.g. Lactobacillus sanfranciscensis) of mature sourdoughs. Enterococcal starter strains produce bacteriocins showing a broad inhibitory spectrum, including pathogenic, toxigenic, and food-spoilage bacteria. Bacteriocins are antimicrobial peptides that inhibit, micro-organisms that are usually closely related to the producer strain – competitive much! The bacteriocins and rapid acidification of the starter environment inhibit the spoilage bacteria (mostly rope-producing bacillus and mold) (Fig. 4).
Establishment of Lactobacillus sanfranciscensis
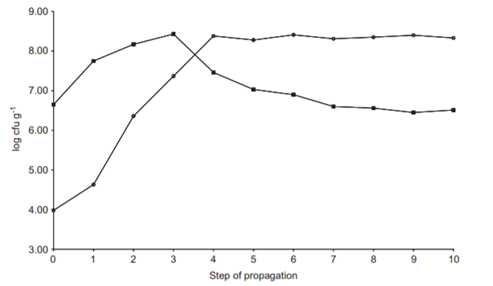
However, once Lactobacillus sanfranciscensis (rod-shaped LAB) have colonized the doughs, it determines a rapid decrement of LAB cocci which subsequently remained at subdominant concentrations (Fig.5). Lactobacillus sanfranciscensis, due to utilization of specific amino acids and peptides, presence of maltose phosphorylase, and pronounced proteolytic activity, is physiologically well adapted to the sourdough environment. This marks the establishment of a mature starter. As the LAB establishes itself over the days and propagations, the pH drops due to lactic acid and acetic acid production. These organic acids also help build a hostile environment for unwanted microbes.
Conclusion
In conclusion, the activity on Day 2 (Fig. 2) of starter creation is abrupt. It mostly portrays the rotting and growth of rouge microbes on the starter! The starter is far from ready. During subsequent propagation, the variety of LAB strains tends to be reduced greatly. Only a few dominant strains survive at the end. The subdominant LAB creates an environment conducive for the dominant LAB species. Firstly, by expressing bacteriocins and most importantly by, rapidly acidifying the starter culture.
These are obscure to the naked eye!
This leads to the establishment of rod-shaped LAB species leading to stabilization of an acidic pH at 3. This also explains the trend of data (Fig. 3) from my stint, abundantly. My graph depicts visual changes only. However, the scientific findings from various studies give a peek into intricate interactions, associations, and reactions happening at molecular levels.
References
Settanni, L., Massitti, O., van Sinderen, D., & Corsetti, A. (2005b). In situ activity of a bacteriocin-producing Lactococcus lactis strain. Influence on the interactions between lactic acid bacteria during sourdough fermentation. Journal of Applied Microbiology, 99(3), 670–681. https://doi.org/10.1111/j.1365-2672.2005.02647.x
Corsetti, A., Settanni, L., & van Sinderen, D. (2004). Characterization of bacteriocin-like inhibitory substances (BLIS) from sourdough lactic acid bacteria and evaluation of their in vitro and in situ activity. Journal of Applied Microbiology, 96(3), 521–534. https://doi.org/10.1111/j.1365-2672.2004.02171.x
Corsetti, A., Settanni, L., Valmorri, S., Mastrangelo, M., & Suzzi, G. (2007). Identification of subdominant sourdough lactic acid bacteria and their evolution during laboratory-scale fermentations. Food Microbiology, 24(6), 592–600. https://doi.org/10.1016/j.fm.2007.01.002
Fraberger, V., Ammer, C., & Domig, K. J. (2020). Functional Properties and Sustainability Improvement of Sourdough Bread by Lactic Acid Bacteria. Microorganisms, 8(12), 1895. https://doi.org/10.3390/microorganisms8121895


Responses